Oxford-AstraZeneca vaccine is approved for UK use

The UK approves its second vaccine against Covid-19. Matt Hancock said “the UK order of 100 million does is enough to vaccinate 50 million people and together with Pfizer-BioNTech jab will cover the entire population. This marked a significant moment in the fight against the virus and 2021 can be a year of hope and recovery because we can see our way out of the pandemic”.
On Tuesday 53, 135 new Covid cases were recorded in the UK the highest single-day rise with 414 more deaths within 28 days of a positive test.
Prime Minister Boris Johnson said “ the vaccine development is a triumph for British science. We will now move to vaccinate as many people as quickly as possible.”
AstraZeneca’s CEO Pascal Claude Roland Soriot said “ the vaccine has a winning formula, the company will progressively ramp up the vaccination program and will be able to deliver up to two million doses a week. “
More than 600, 000 people in the UK have been vaccinated since Margaret Keenan became the first in the world to be given it outside of a clinical trial.
Professor Andrew Pollard, director of the Oxford Vaccine Group said “the vaccine approval was an astonishing achievement in science and clinical research. But there is still more work to do, It’s not over yet. Our colleagues in the hospital are facing some real horrors caused by the virus. The next steps are critical”.
The Oxford vaccine is injected into the patient. Scientists took genes for the spike protein on the surface of the coronavirus and put them in a harmless virus to make an Oxford vaccine. The vaccine enters the cells which then starts to produce the spike protein. The body’s immune system reacts, produces antibodies, and activates T-cells to destroy cells with the spike protein.
If the patient later catches coronavirus, antibodies, and T-cells are triggered to fight the virus.
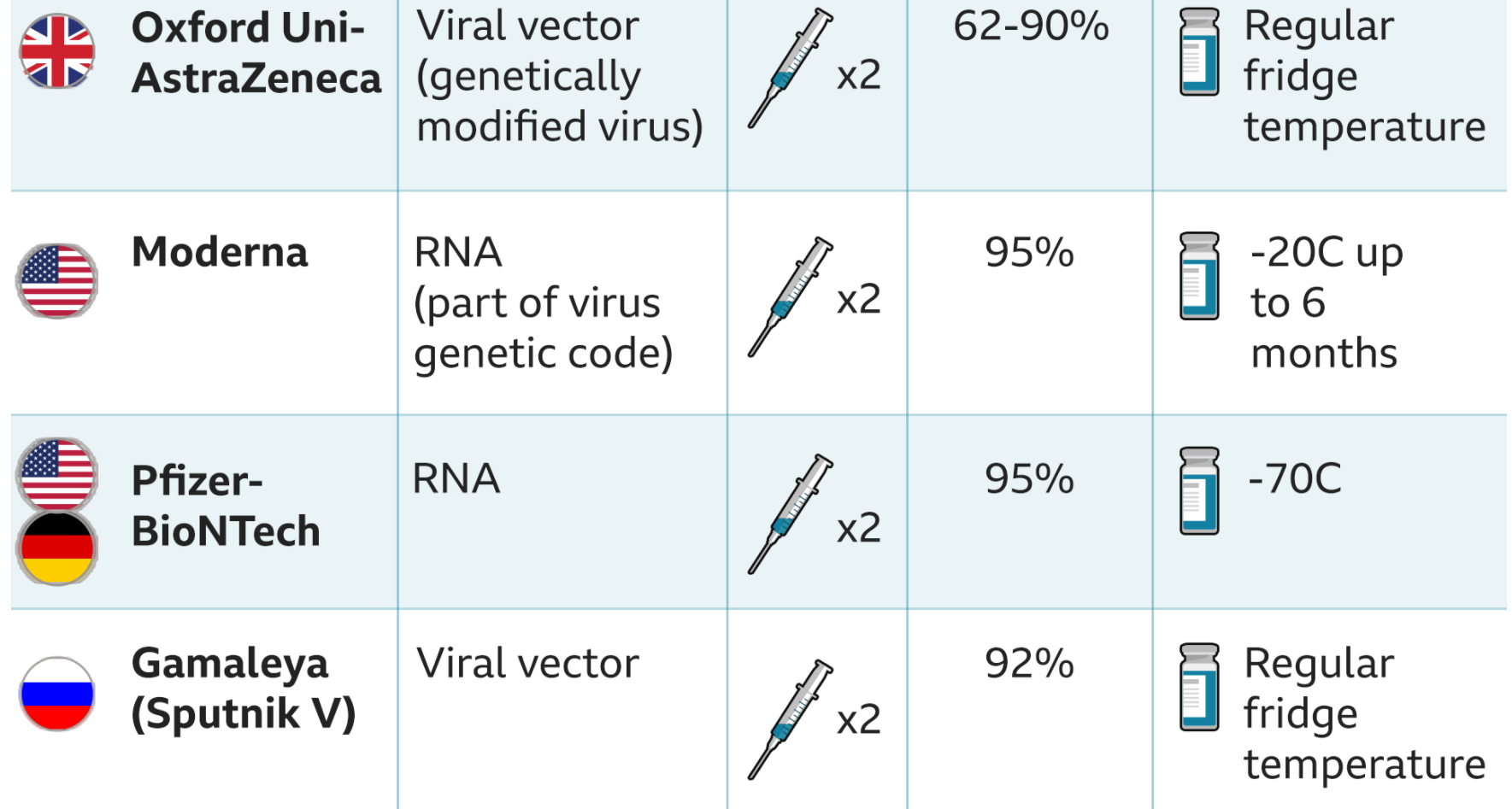
The first analysis of trial data showed 70 per cent of people were protected from developing Covid-19 and nobody developed the severe disease or needed hospital treatment.
The figure was just 62 per cent when people were given two full doses of the jab and 90 per cent when they were given a half dose and then a full one.
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved two full doses of the Oxford-AstraZeneca vaccine.
Unpublished data suggest that leaving a longer gap between the first and second doses increases the overall effectiveness of the jab.
The vaccine works in the elderly who developed high levels of antibodies and T-cells after they were vaccinated. The Oxford vaccine costs £3 a dose and can be stored in a fridge, a third of its Pfizer/BioNTech rival.