Pfizer-BioNTech Covid vaccine offers 90 per cent protection

Pfizer and BioNTech have tested their first effective coronavirus vaccine on 43. 500 people spanning six countries and so far no safety concerns have been raised. Preliminary analysis reveal the vaccine can prevent more than 90 per cent of people from getting Covid-19.The companies plan to apply for emergency approval to use the vaccine by the end of the month said: “ great day for science and humanity”.
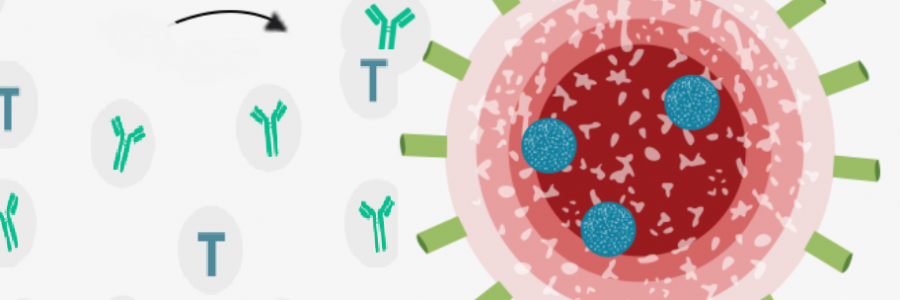
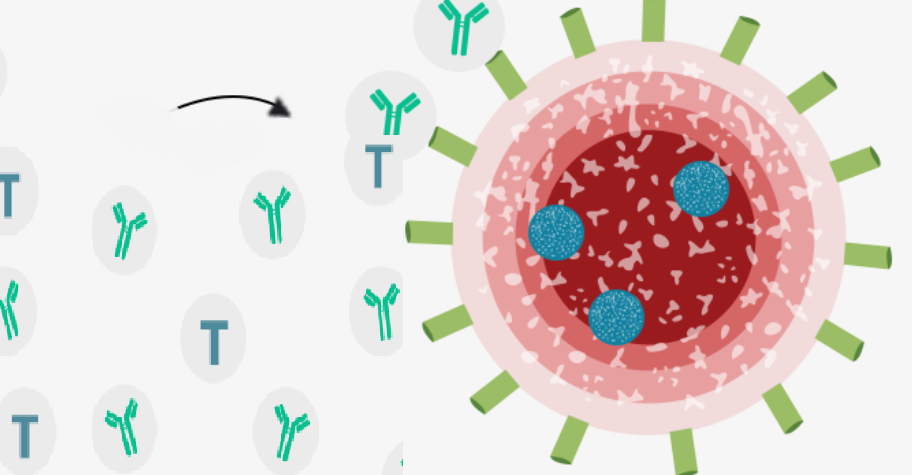
There are around a dozen in the final stages of testing – known as phase 3 trial – but this is the first to show any results, by using a completely experimental approach that involves injecting part of the virus’s genetic code in order to train the immune system. Scientists take part of the virus’ genetic code or RNA that tells cells what to build, and coat them in a lipid so they can enter the body’s cells, which is injected into the patient. The vaccine enters the cells and tells them to produce the coronavirus spike protein which prompts the immune system to produce antibodies and activate T-cells to destroy infected cells. If the patient encounters coronavirus the antibodies and T-cells are triggered to fight the virus.
Two doses, three weeks apart are needed. The trials in US, Germany, Brazil, Argentina, South Africa and Turkey show 90 per cent protection is achieved seven days after the second dose.
Pfizer believes it will be able to supply 50 million doses by the end of 2020 and around 1.3 billion by the end of 2021.
The logistical challenges are as the vaccine has to be kept in ultra-cold storage at below -80ᵒC.
The companies are yet to present a breakdown of the vaccine’s effectiveness in different age groups and how long immunity lasts.
Dr. Albert Bourla, the chairman of Pfizer said: “ We are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis.” Prof Ugur Sahin, one of the founders of BioNTech said “ results are a milestone”.
The UK has already put in an order for 40 million doses enough for 20 million people.
