UK First in the world to approve Pfizer/BioNTech Covid vaccine for roll out next week
The UK has become the first country in the world to approve the Pfizer-BioNTech coronavirus vaccine by the British regulator, the MHRA, who says the jab, which offers 95 per cent protection against Covide-19 illness I safe for rollout.
The UK has already ordered 40 million doses enough to vaccinate 20m people with two shots each. Immunisation can begin within days for people with high priority groups as the first doses of 800, 000 manufactured in Belgium, would be arriving in the UK in the coming days.
The approval of the vaccines is fast-tracked to go from concept to reality, taking only 10 months to follow the same developmental steps that normally span a decade.
Health Secretary Matt Hancock tweeted saying: “ The NHS stands ready to vaccinating early next week.”
Although vaccination can start people are warned to remain vigilant and follow coronavirus social distancing rules, wearing face masks, and testing people who may have the virus, and asking them to isolate.
Health Secretary Matt Hancock has also claimed Brexit allowed the UK to approve a Covid vaccine more quickly other than European Union (EU) countries. “We do all the same safety checks and the same process, but we have been able to speed up how they’re done because of Brexit”. The Leader of the House of Commons Jacob Rees-Mogg tweeted, “We could only approve this vaccine so quickly because we have left the EU.”
The European Medicines Agency (EMA) which is in charge of approving the vaccine in the EU, said it had the “most appropriate” method to approve the vaccine, as it studies data from lab studies and large clinical trials. ” These are essential to ensure a high level of protection to citizens during the course of a mass vaccination campaign”. Under EU law countries can evoke emergency powers to temporarily approve a vaccine in the event of a pandemic. The UK, still a member of the EMSA, was able to approve the vaccine under this rule, despite suggestions from ministers that Brexit had enabled prompt approval.
America’s Food and Drug Administration does have a different approach to other regulators around the world as if often asks vaccine makers for their raw data, which it then spends time re-analysing. The UK’s medicines regulator in London relies more heavily on the companies’ own report as does the European Medicines Agency based in Amsterdam.
The FDA said it wanted to see two months’ extra safety data from the final phase vaccine trials before pharmaceutical companies could apply for emergency approval. The US has got bogged down in a much more detailed review than might have been necessary.US having surpassed 14.5 million Covid-19 infections with a recorded 283, 000 deaths, while the UK surpassed 1.7m Covid-19 infections with a recorded 60, 113 deaths.
UK’s Chief medical officer Prof Jonathan Van-Tam said “ MHRA has 100 yeasts of medical experience between the UK regulator and the committee advising which groups of people are vaccinated first.
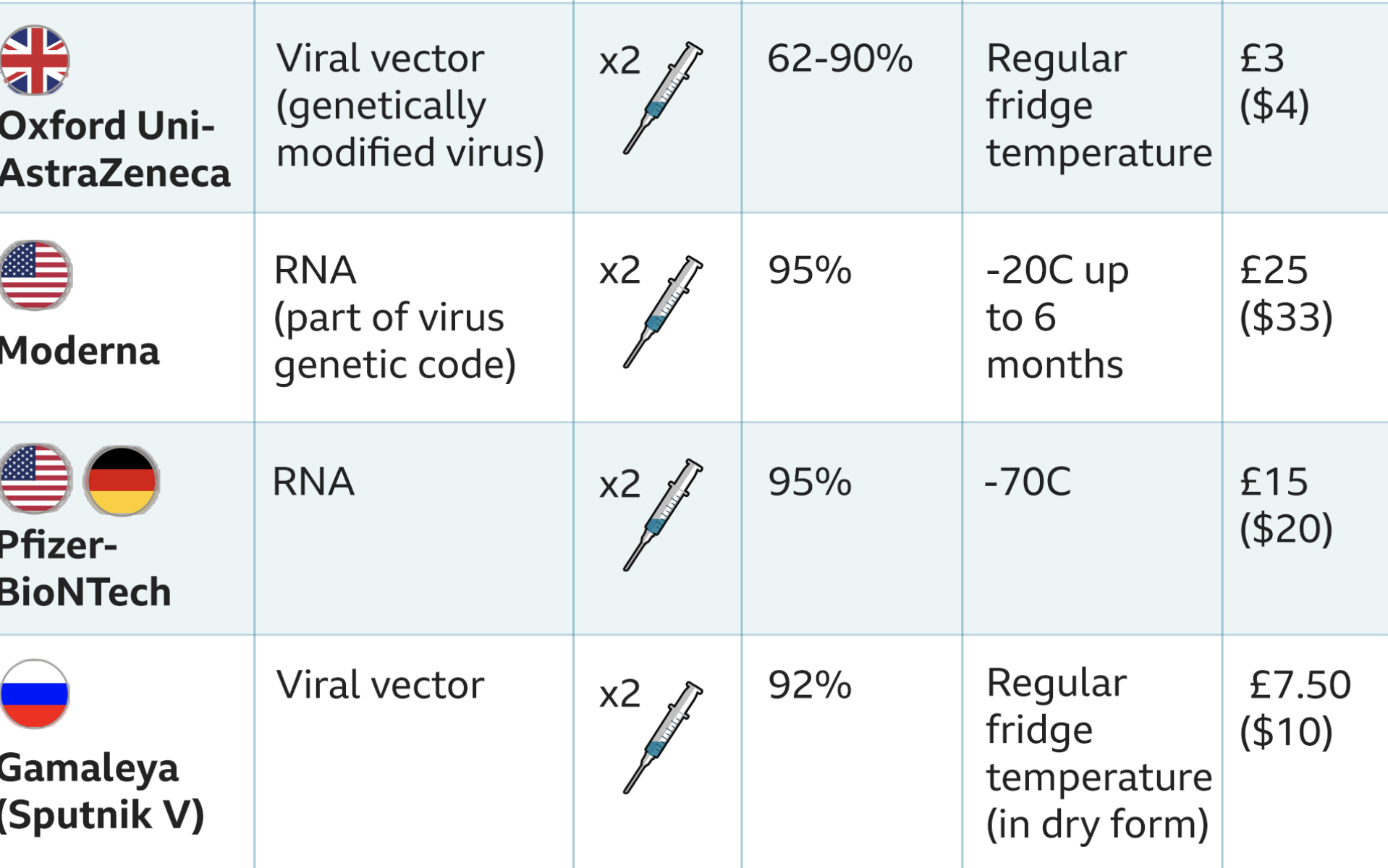
Although no mRNA vaccine has never been approved for use in humans, the new approved type of mRNA vaccine uses tiny fragments of genetic code from the pandemic virus to teach the body how to fight Covid-19 and build immunity.
Scientists take part of the virus’ genetic code or RNA, that tells cells what to build, and coat them in a lipid so they can enter the body’s cells.
The vaccine must be stored at around -70°C (-94°F) and will be transported in special boxes of up to 5, 000 doses packed in dry ice. Once delivered it can be kept for up to five days in a fridge. And once out of the fridge it needs to be used within six hours.
Mass immunisation of everyone over 50, as well as younger people with existing health conditions, can happen as more stocks become available in 2021. The vaccine which costs £15 per dose is administered as two injections, 21 days apart, with the second dose being a booster.