Life could exist in Mars and Titan
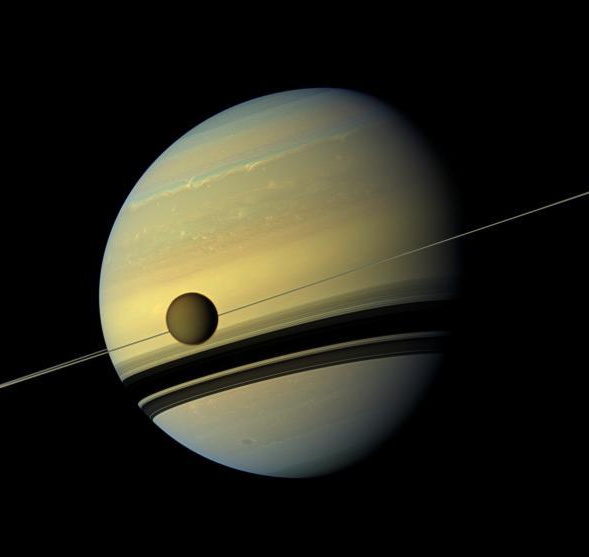
Titan-pictured-infront-of-Saturn-by-Cassini-NASA-jet-propultion-lab.
Saturn’s largest Moon Titan, life might exist beyond the bounds of water-based chemistry, according to scientists at Cornell University. According to a report in the latest issue of the Proceedings of the National Academy of Sciences, the researchers found Hydrogen Cyanide (HCN) in the planet’s atmosphere and speculate that it could become a possible prebiotic chemical key which can react to form long chains or polymers, one of which called Polyimine. Huygens mission and data collected by NASA’s Cassini researchers have revealed that under Titan-like cold environmental conditions, polyimine is flexible and can absorb the sun’s energy and become a possible catalyst for life.
Titan is a very cold place and instead of water on the surface, it is filled with liquid methane and ethane. Its dense atmosphere, a yellow haze, is full of nitrogen and methane. When sunlight hits this toxic atmosphere, the reaction produces hydrogen cyanide.
Due to its distance from the Sun, Titan’s surface temperature is about 90 K (−179 °C, or −290 °F). At these temperatures, water ice—if present—does not melt, evaporate or sublime, but remains solid and extreme cold and also lack of carbon dioxide (CO2) in the atmosphere.
“We are used to our own conditions here on Earth, our scientific experience is at room temperature and ambient conditions. Titan is completely different beast” said martin Raham, postdoctoral researcher in chemistry at Cornell University and lead author of the study. “ So if we think in biological terms, we’re probably going to be at a dead end.”
Still Titan and Earth have important traits in common. Despite its seemingly inhospitable climate, Titan features terrain with Earth like attributes such as lakes, rivers and seas. These liquids fall as rain and affect geology through erosion. “This paper is a starting point, as we are looking to prebiotic chemistry in conditions other than Earth’s, we need to continue to examine this, to understand how the chemistry evolves over time. We see this as a preparation for further exploration” Raham said.
