Volta 270th Birthday!

The World Celebrates the 270th Birthday of Volta the inventor of Battery and volts. Volta from Como northern Italy, born on 18 February 1745, married in 1794, to Teresa Peregrini, and raised three sons: Flaminio, Zanino and Luigi. In 1774, invented and developed the Electrophorus, a device that produced static electricity. He researched and discovered methane methane at the Lake Maggiore and by 1778 he managed to isolate methane. He devised ignition of methane by electric spark in a closed vessel and also studied electrical captacitance developing separate means to study both electrical potential (V ) and charge (Q ), and discovering that for a given object, they are proportional. This is called Volta’s Law of Capacitance and it was for this work the unit of electrical potential has been named the Volt.
In 1779 he became a professor of experimental physics at the University of Pavia, a chair that he occupied for almost 40 years. He also discovered the electrochemical series and law of electrical force (emf) of a galvanic cell, comprising of a pair of metal electrodes, separated by electrolyte, is the difference between their two electrode potentials (thus, two identical electrodes and a common electrolyte give zero net emf). This may be called Volta’s Law of the electrochemical series.
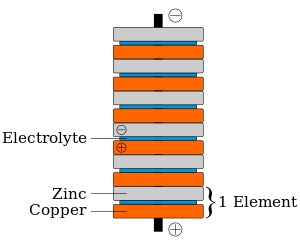
In 1800, Volta invented the voltaic pile, an early electric battery, which produced a steady electric current.Volta found the most effective pair of dissimilar metals to produce electricity Zinc and Silver after he experimented with individual cells in series, each cell being a wine goblet filled with brine into which the two dissimilar electrodes were dipped. The voltaic pile replaced the goblets with cardboard soaked in brine.
The battery made by Volta is credited as the first electrochemical cell. It consists of two electrodes: Zinc and copper. The electrolyte is either sulphuric acid mixed with water or brine. The electrolyte exists in the form 2H+ and SO42−. The zinc, which is higher in the electrochemical series than both copper and hydrogen, reacts with the negatively charged sulphate (SO42−). The positively charged hydrogen ions (Protons) capture electrons from the copper, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode and an electric current will flow if they are connected. The chemical reactions in this voltaic cell are as follows:
Zinc:
Zn → Zn2+ + 2e−
Sulphuric acid:
2H+ + 2e− → H2
The copper does not react, and it functions as an electrode for the electric current.
However, this cell also has some disadvantages. It was unsafe to handle, since sulphuric acid, even if diluted, which can be hazardous. The power of the cell diminishes over time because the hydrogen gas is not released. Instead, it accumulates on the surface of the zinc electrode and forms a barrier between the metal and the electrolyte solution.